Maximize Value with Surplus Sodium hypochlorite in Disinfectants & Water Treatment
Sodium hypochlorite, commonly known as bleach, is a powerful and versatile chemical solution widely used for disinfection and water treatment. This chemical, available in various concentrations, is celebrated for its strong oxidizing properties and efficiency in eliminating harmful microorganisms. When held in surplus, this chemical represents more than just an overstocked resource; it is an opportunity. Companies with surplus inventory can recover significant costs by selling unused or excess stock, turning a potential liability into a profitable asset. The industrial relevance of Sodium hypochlorite in both routine disinfection and emergency water treatment further emphasizes its importance in maintaining public health and safety.
Buy and Sell Surplus Sodium hypochlorite: Transforming Disinfectants & Water Treatment
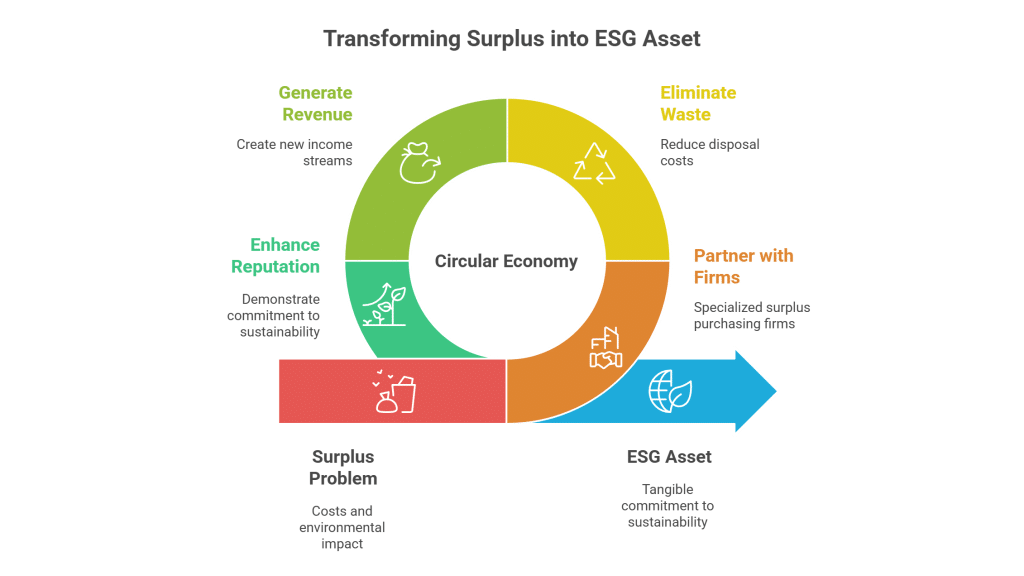
The trading of surplus chemicals like Sodium hypochlorite offers tremendous value for both buyers and sellers alike. Sellers can free up vital storage space, avoid costly disposal fees, and even earn revenue on chemicals that would otherwise incur disposal expenses and regulatory burdens. On the other hand, buyers benefit from reduced procurement costs, reliable sourcing, and enhanced sustainability credentials by using chemicals that might otherwise contribute to waste. Moreover, surplus trading supports environmental stewardship by ensuring that chemicals are used efficiently and safely, thus minimizing negative impacts on our surroundings. In many cases, companies not only save money on waste management but can also profit directly, further promoting responsible resource management.
Sodium hypochlorite in Disinfectants & Water Treatment
For buyers, surplus Sodium hypochlorite offers cost-effective acquisition with performance reliability. Purchasing surplus stocks means access to high-quality disinfectant solutions at reduced costs while maintaining supply chain continuity. Buyers can benefit from bulk purchase discounts, rapid procurement, and reduced lead time ensuring operational consistency in both routine and emergency water treatment scenarios.
Sellers gain significant financial and logistical advantages by offloading surplus Sodium hypochlorite. By converting excess inventory into revenue, companies can optimize storage facilities, lower disposal costs, and adhere to environmental regulations. This trading process not only recovers costs but also supports sustainable practices by preventing unnecessary chemical waste, further enhancing the manufacturer’s market reputation.
Table of Contents
Municipal Water Treatment Plant Transforms Surplus into Strategic Savings
A major municipal water treatment facility recently faced the challenge of managing surplus Sodium hypochlorite stock. Instead of incurring high disposal costs and storage fees, the plant opted to trade its excess inventory on a surplus chemical platform. This move allowed the facility to secure additional funds, streamline its inventory management, and maintain a reliable supply of disinfectant for emergency use. By integrating surplus trading into their procurement strategy, the plant not only ensured continuous water safety but also met important environmental compliance standards, setting an example for sustainable chemical management across the industry.